
“It is the function of science to discover the existence of a general reign of order in nature and to find the causes governing this order. And this refers in equal measure to the relations of man – social and political – and to the entire universe as a whole.”
Dmitry Mendeleev
From the beginning of time it has been a human fascination to understand the matter that surrounds us. First it was created by a higher power for all our needs, to today when we know so much more about about not just the things we can see but also the things that are hidden from our view. The very nature of what we can’t see has such a profound impact on the world around us and this is what I have been studying for the past few months of science 9.

As you go down the list of definitions you should have a basic understanding of the things we learned this term. All the definitions were terms we learned about.
Periodic Table
A graphical representation of the groupings of atoms. This graphic displays the families in which these elements, and many other details about the atoms themselves.
Molecule
An Atom or group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction.
Element Families
There is a number of element families in the periodic table all sharing the the same number valence electrons (helium is the only outlier because it’s maximum 2 electrons in its valence shell)
Alkali Metals (1 valence electrons)
A group very reactive and soft metals found on the very left of the periodic table. These metals tend lose one electron when bonding
EXAMPLE – Sodium
Alkaline Earth Metals (2 valence electrons)
A group of moderately reactive metals found one collumn to the left on the periodic table
EXAMPLE – Calcium

Transition (workforce) Metals (2 valence electrons)
A group of majority stable, dense, hard metals that spans from column 3 to 13 on the periodic table
EXAMPLE – Nickel

Actinide and Lanthanide Metals (2 valence electrons)
A expansion from inside the transition metals. All the actinides have no stable isotopes and are radioactive. However, all but 1 lanthanide has a stable isotope. Both groups are very similar to each other with many similar characteristics.
EXAMPLE – Lanthanum
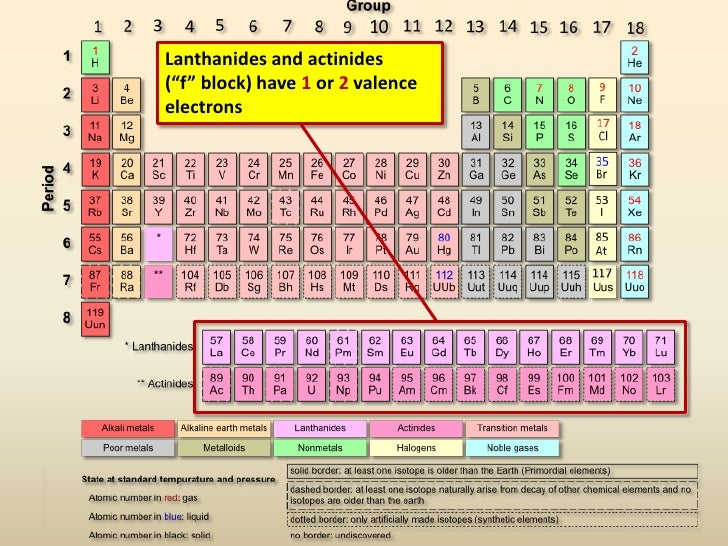
*** SKIPPING OVER A FEW GROUPS ***
Halogens (7 valence electrons)
A group of reactive non-metals that form both covalent and ionic bonds very commonly. These elements are very reactive.
EXAMPLE – Fourine

Noble Gases (8 valence electrons)
Very very stable non-metals which do not bond with any other element.
EXAMPLE – Helium
Element
A very similar group of atom(s) that all have the same amount of protons. Variations can occur in both neutron counts and electron counts but the number of protons remains constant.
EXAMPLE – Hydrogen
Atom
A atomic particle that is made up of electrons, protons, and majority of the time neutrons. These particles can bond with each other to create larger molecules. These particles can be displayed in Bohr models.
EXAMPLE – Fe (III)

Electron
A negatively charged subatomic particle that can be found orbiting the outside of the nucleus of an atom.
Proton
A positively charged subatomic particle that can be found in the nuclei of every atomic particle. The charge is equal in force to that of an electron.
Neutron
A neutrally charged subatomic particle that is about the same mass of the proton and is also found in the nuclei of an atom
Bohr Models
A model of an atom that displays the number of protons and neutron in the nucleus and the number of electrons in each shell. These models can also be used to show the process of covalent and ionic bonding.

Bonded Atoms
A body of two or more atoms that have been bonded together covalently or ionically.
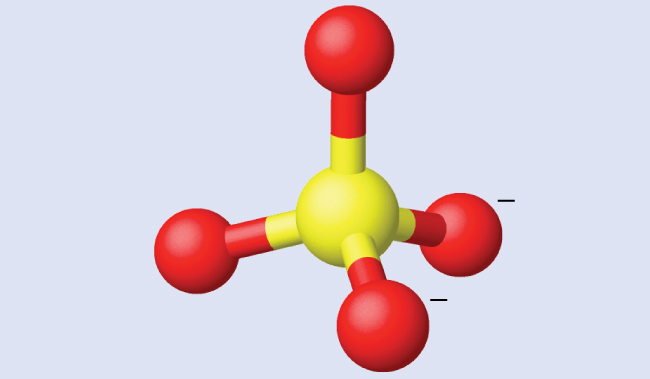
Covalent Bond
A bond in which atoms share electrons in their respective valence shells, this sharing will create a full valence shell for all atoms involved.
Ionic Bond
A bond in which atoms give one electron to the other(s) to fill out their valence shells. The bond occurs because the atoms because of their respective ionic charges.
Valence Shell
The outermost shell of electrons in an atom. This outermost shell is where all atomic bonding occur at whether covalently or ionically.
Ionic Charge
The charge of an atom. It can be determined by comparing the count of protons and electrons in the atom.
In the final stage of the unit we used all what we had learned and made it into a chemistry video that, showed the concepts we learned.


