
Fair warning, before this begins, I have found a series of science gifs and memes to use. You have been warned
So, in case you can’t tell, we did a chemistry unit! This involves a lot of things that I didn’t know a lot about. I know the basics of the periodic table, and what an atom is, but not much else beyond that. Though, I really enjoyed this unit. Though I can’t name specifically what I liked, it was really fun to do.

We learned a lot during this unit, so let me break it down. For starters, we studied the periodic table, along with Bohr diagrams, Lewis structures, and how to do an experiment.
Bohr Diagram
A Bohr diagram is an easy way to show off what the atom of an element looks like. Since we did a lot of work, I’ll show you one of my drawings:
Lets use the Be (Berilluim) as an example. You see how there’s a tiny 4 beside it? That’s how many electrons are in the atom. The rings around the atom are called ‘valence shells’. The closest valence shell can only hold 2 electrons, and the next one after than can hold eight, and then the rest can also only hold eight. So, the Berilluim diagram shows two electrons on the innermost valence shell, and two more in the outer, because there are four electrons.

Lewis Structure
Onto Lewis diagrams. The diagram below shows Four hydrogens bonding with carbon
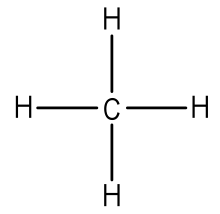
I feel like that’s fairly simple to understand. The hydrogen and the carbon are sharing electrons, so they’ve bonded.
Lab Procedure
This has a bit more to do with our project, that I’ll explain further down. So, you need to start off a lab with a driving question. Let me use an example we did in class: is there Hydrogen in our breath?
Next, you created a solid hypothesis: Yes, there is Hydrogen in our breath. Obviously there is, but we’re doing an experiment to prove that.
Then, you need your materials. The materials we used were a straw, a beaker, some water, and some bromothymol blue, which turns yellow if it detects acid. Acidic things are acidic due to hydrogen, so the Bromothymol Blue should turn yellow if there’s hydrogen in our breath.
Create a procedure that’s simple and productive. What we did for this was put some of the B.B. (I abbreviated it, so sue me) in water, and then blew air through the straw into the solution.
Then there was the conclusion. We concluded that there was, in fact, hydrogen in our breath because the solution turned yellow, as it does when mixed with hydrogen.
Very attractive.
The Project
To finish off the unit, we had to do a project. We had two choice: created an animated video to show how atoms bond, or create a lab procedure. My group (Hannah and Willa) chose the latter. Let me show you our lab procedure:
Hypothesis for Project:
We think that there will be different levels of acid in each substance.
Materials Needed:
– Bromothymol Blue
– Dirt
– Banana
– Coca Cola
– Pineapple
– Bath bomb
– Beaker
– Water
Theories of acid levels (highest to lowest)
– Pineapple
– Coke
– Bath bomb
– Banana
– Dirt
Actual Ph levels:
-Coke
-Pineapple
-Banana
-Dirt
-Bath Bomb
Cabbage Juice Explanation:
When the cabbage juice reacted with the base (bath bomb), OH particles attached onto the chemicals in the cabbage juice, which caused it to turn a green colour, indicating that it was a base.
When the cabbage juice reacted to any of the acids (pineapple, banana, coke), it gave hydrogen to the anthocyanin, which caused it to turn more and more red depending on how low the pH was.
The anthocyanin in the cabbage juice is what reacts with the H+ and the OH in different products, which in turn causes them to change colour depended on how high or low the pH in each product is. If the pH is below 7, it turns red or pink. If the pH is exactly 7, it will not react, because it’s neutral. If the pH is above 7, it will turn green or yellow.
This is the anthocyanin without having reacted with anything
This is the anthocyanin after having reacted with a base. As you can see, an OH particle has attached on, causing it to turn green.
This is the anthocyanin with acid. It have more hydrogens, causing it to turn pink.
Chemical formulas for acids:
Pineapple: Ethyl butyrate
C6H12O2
Cola: Coca Cola
C30H32N8O10
Banana: Isoamyl acetate
C7H14O2
Chemical formula for base:
Bath bomb: Sodium bicarbonate
NaHCO3
Chemical formula for neutral:
Dirt:
There’s nothing in it to make it acidic or basic
Indicator:
Red Cabbage: Anthocyanin
C15H11O+
Conclusion:
As you can see, all of the acids have some amount of Hydrogen in them. The cola has 32 hydrogens, which shows that it’s the most acidic, and the pineapple has the least, with 12. The sodium bicarbonate in the bath bomb made it basic. The dirt, having nothing in it to make it acidic or basic, was neutral.
Now, this is all fine and dandy, but what was the actual product? Well, I’m glad you asked. (Yes, I understand that I asked for you, but just go with it)
We created a video of our experiment, which turned out really cool:
So, to conclude this unit, we made two mind maps. One was at the start, about what we knew, and then another at the end, to compare what we learned:
Thats all for this post
Read Ya Later
Sincerely, Me








