
Although they can happen on a scale so small we sometimes can’t see them, chemical reactions can have big consequences. In our latest unit, we learned all about different chemical reactions, how they work, and what the consequences of their occurrence may be.
What is a Chemical Reaction? – What we Learned
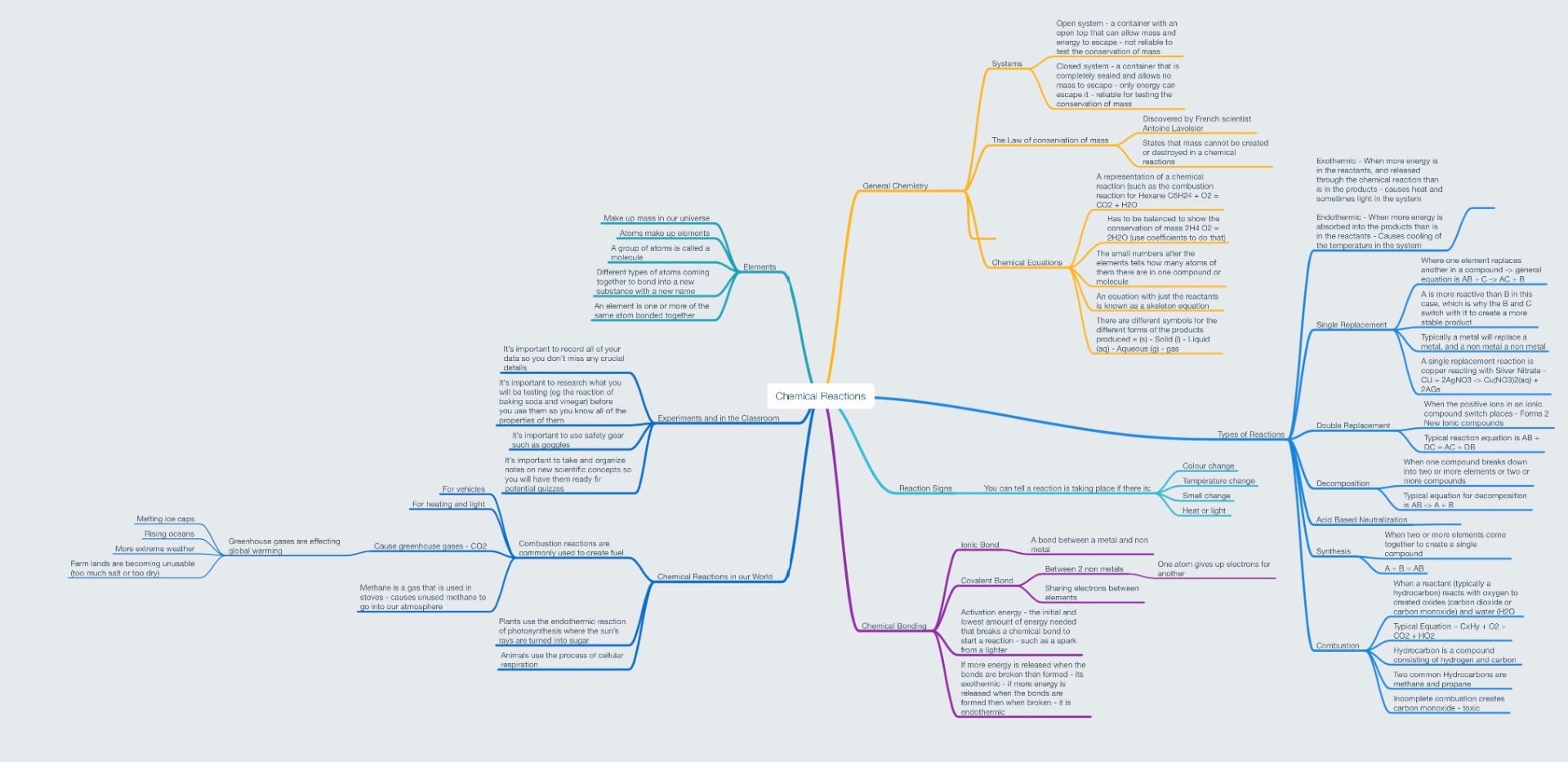
A chemical reaction is when the molecules in a compound (either Covalent or Ionic), are rearranged and create new new compounds or elements. They occur when enough activation energy is provided to reactive substances (substances that want to become more stable) and can produce a variety of products such as heat, light, water, or gas just to name a few. There are 6 different types:

Chemical reactions can be displayed in something known as a chemical equation. To write one, you start off with a skeleton equation:

This particular one is for Methane Combustion, which is an exothermic reaction. This means that more energy is released from the reactants than is stored in the products. If it were the opposite way around, it would be known as a endothermic reaction. This skeleton equation is a great place to start, but it does not represent the conservation of mass, or the law that:

The law of the conservation of mass means that in a closed system (a system that mass cannot escape from, but energy can) the mass must stay constant throughout the reaction, and must be represented in the chemical equation. To fix this, we must balance the chemical equation by writing it as:

Our Project – Chemical Reactions in our World
Chemical reactions don’t just occur in the lab; they are happening all around us. Plants use the endothermic process of photosynthesis in which they absorb the sun’s light to create sugar, and animals use the exothermic process of cellular respiration to turn this stored energy into heat and fuel. There are also many man made chemical reactions that we see on a daily basis, such as the combustion reactions used to fuel vehicles and create electricity. Unfortunately, these reactions create the harmful product of greenhouse gases, which contribute to global warming. Our project aimed to answer the question of:

You can watch our presentation video below:
The Experiment
In our video, we outline that the main way we came to our conclusion was through an experiment in which we simulated the greenhouse effect in a controlled environment. We got this idea from the Myth Busters episode below:
In our video, we go into greater detail about the process of our experiment, but I have outlined the basic scientific method we went through to conduct it:

Reflection
Our project included many of the curricular competencies, but focused around these main four (click the tabs to read more):
My Project End Mind Map
Due to a reset of MindNode, I no longer have access to my unit start Mind Map, but I have summarized my learning in the Unit End Mind Map below:
My Personal Learning
I’m always talking about how PLP projects provide such good learning experiences, but I really mean it with this one. Like I said earlier, chemistry isn’t always intuitive, which can be a challenge for me. I typically learn by going off and figuring stuff out on my own, and sure that works in humanities and other classes, but I can’t do that as much in science. I need to accept help, and not just in answers to the questions I ask, but in things that I may not always reach out about. I now realize that this project was a good opportunity for me to improve upon this because the labs forced me to consider the methods of others. I needed to bring my learning to a more interdependent level, and I actually did better at this as the project went on. I didn’t realize it at the time, but by doing this I was also improving upon the competency of Collaboratively and individually planning selecting and using appropriate investigation methods, including lab experiments.
Between the labs, the explosions, and the fascinating research, I have to say this has been one of the funnest science units I’ve ever done, and I can’t wait to see what we are doing next.

Hello Emily, on the post of World War 11 in Canada, l have with a lot of interest read how Canada was part of the war. this was a detailed work. Whereas Kenya at that time was not at war, but as a British colony, Kenyans went to fight for the British in far countries as Burma, India, in the army, as cooks, drivers and so on.
Hello Thomas,
Thank you for your comment! I am glad that you enjoyed my post, and it’s interesting to hear about the effect of the war in other countries.
Emily J