The project we finally just completed in our Sciematics 8 class was called “Chemistry Coding”. This project was all about atoms, molecules, density and states of matter. These topics can all be classified into 2 big fields. Kinetic molecular theory, and atomic theory. These were the main subjects that we researched during this project. The driving question for our project to guide us through was “how is the motion of atoms and molecules related to temperature”. To show our learning and understanding of the driving question, we had to code a game applet showing kinetic molecular theory and atomic theory using an easy coding software called scratch. Scratch is a really simple, fun and easy way to learn coding so it was the perfect software to jumpstart this project.
My first recollection of learning kinetic molecular and atomic theory starts in when we learned about the solid sphere model and all the different atomic models throughout history. The first one that we learned about was in 1803 that John Dalton first produced seen in the picture below. John Daltons model pictured a solid sphere much like a billiard ball. He stated that the atoms were so small that they were indivisible which means they could not be broken down any more. This theory turned out to be incorrect. In fact, J.J. Thompson proved this theory wrong. We also learned that John Dalton stated how atoms of the same element were identical, while compounds were in fact multiple atoms built up to make one substance. This theory turned out to be true!
Throughout this project we learned about all the different atomic models throughout history. The big one that stuck for me was J.J. Thompsons plum pudding model of the atom. The main reason that this model stuck was cause I found J.J.s name funny and the model was also very simple so I could remember the specific details. J.J. released this much more accurate model of the atom in 1904. We learned how the electrons were scattered throughout the atom. We learned this to be a big milestone in atomic research.
We also learned about the third big model in atomic research history, created by none other than Ernest Rutherford. His model represented the nucleus and was first released in 1911. This model represents electrons orbiting around the nucleus. Rutherford experimented and became quite famous in the science world for when he fired positively charged alpha particles at a thin sheet of gold. He concluded that it was only possible for the alpha particles to reflect of the gold sheet it there was a nucleus in the middle of the atom itself. Although he had a big discovery of the nucleus, he could not explain how electrons remain in orbit around the nucleus.
Next was Niels Bohr. In 1913, Bohr clarified Rutherfords model of the atom stating that electrons moved around the nucleus in orbits of fixed sizes and energies. His theory also had a problem though. Electrons should not emit energy and collapse into the nucleus.
The last model we looked at was by Erwin Schrödinger. He created the Quantum Model in 1926 shown below. Schrödinger said that electrons do not move around in set paths around the nucleus, but instead have “clouds of probability” which are more likely to find an electron. This model is still widely accepted as the most accurate model today.
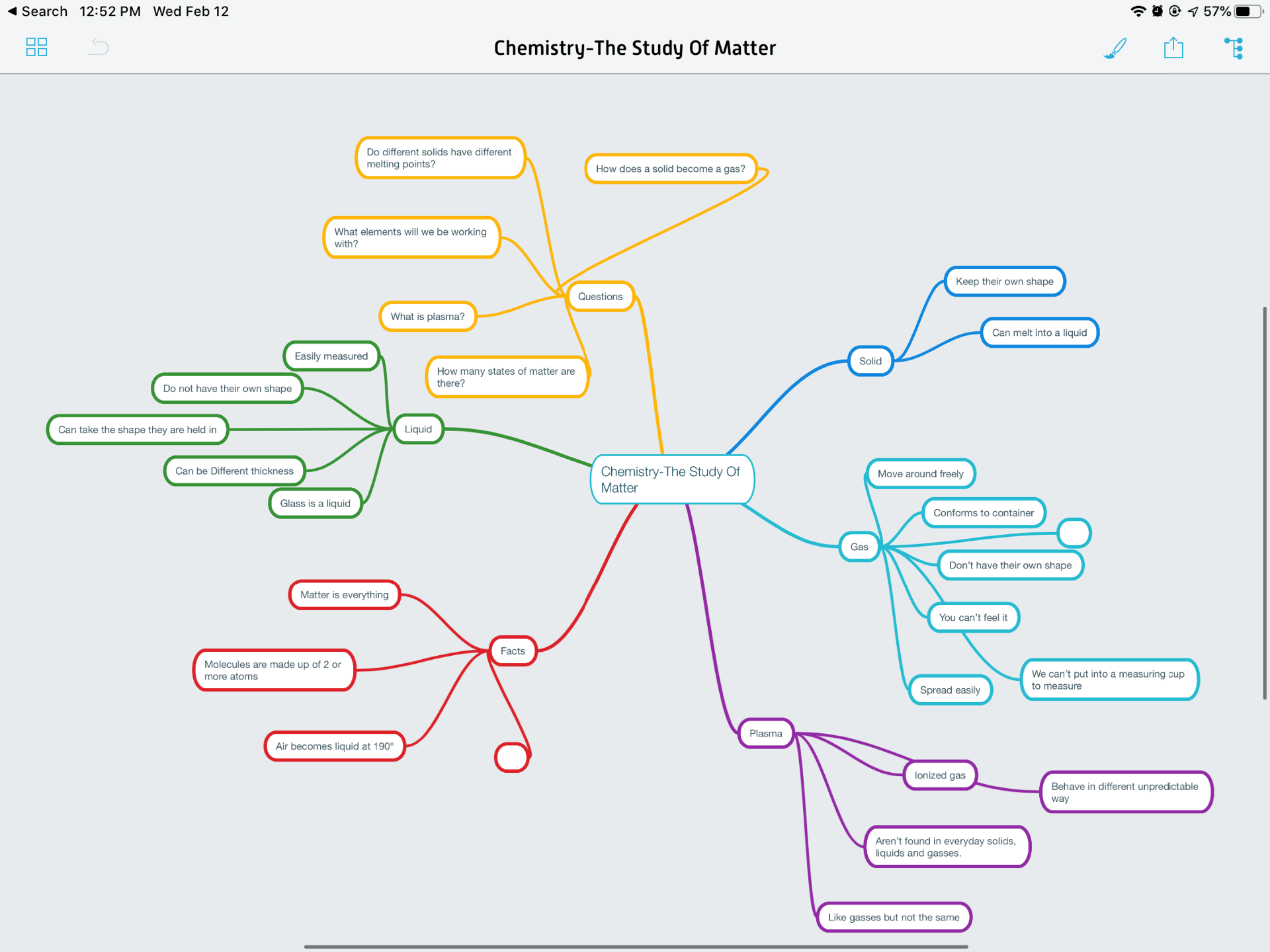
At the start of this project, we created a mind map like every other Sciematics project. Here below you can see how my mind-map and learning knowledge changed from the start of the project, to the end of the project. I answered all my questions thoroughly to show my learning.
The end milestone in this project was coding a scratch game applet to show our learning throughout the project. I with a couple other classmates, chose to code an atom simulator displaying the kinetic molecular theory and the specific behaviour of atoms and molecules. My scratch game in particular displayed my advanced understanding of the competencies and overall learning. It included a pump to pump out the atoms, then the atoms would bounce around a box. The player could heat up the atoms and make them go faster of could cool down the box which would cause the atoms to slow down. The game would also tell you the states of matter and different atoms. The link below is to the game.
Next, to talk about the curricular competencies being assessed in this project. The first one was using our time in class to be proficient in our studies and use the time to be efficient. Throughout the project I think I missed one day where I was on the school ski team field trip. Otherwise, I was there each day, using my time to the best of my ability most of the time*. I still do need to improve the usage of my time in and outside of the classroom and not procrastinate until the very last minute. I think I was proficient in this competency.
The second of the three competencies was scientific communication, or in other words, communicating my learning well through the scratch game applet. I think I was mostly proficient in this category. I showed my understanding of kinetic molecular theory and showed multiple different atoms and molecules. I communicated the different states of matter, showing a gas state, a liquid state and a solid state in the form of a picture even with a label just so the viewer could understand what was happening. I also showed particle motion in a hot environment, a room temperature environment and a cold environment to get the point of solids, liquids and gasses across. Overall I think I extended in this category.
The third and final competency was reasoning and analyzing or in other words, coding an interactive scratch applet. I think I was proficient in this category in the form of my overall code and how streamlined and smooth the simulator was. I had easy logical control that I even labeled in the background to make it easier for the user. Although I had multiple different controls, they all made sense. Reset was the R key, temperature up was the right key, temperature down was the left key, and to pump out atoms was the space bar. Overall I think I created a proficient interactive scratch applet that was easy for the user to play.








Leave a Reply